-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaproLékaře.cz / Odborné časopisy / Česká a slovenská neurologie a neurochirurgie / 2018 - Suplementum 1The factors of pressure ulcer’s healing in critically ill patients
Faktory hojenia dekubitov u kriticky chorých pacientov
Cieľ:
Identifikovať a analyzovať faktory ovplyvňujúce priebeh hojenia dekubitov u kriticky chorých pacientov, následne porovnať variabilitu hojenia dekubitov po stabilizácii zdravotného stavu.
Súbor a metodika: Komparatívna multiprípadová štúdia s využitím kvalitatívnych aj kvantitatívnych výskumných metód. Výskumnú vzorku tvorili traja kriticky chorí pacienti s dekubitom.
Výsledky: Kritický stav nastal po terapeutickom zákroku na srdci (prípad A, C – kardiochirurgický zákrok; prípad B – rádiologický intervenčný zákrok). Dekubity vznikli počas riadenej a podpornej ventilácie, kontinuálnej intravenóznej sedácii a analgézii. K nehojeniu dekubitov významne prispievali hypoxia, hemodynamická instabilita, podávanie vazopresorov s vazokontrikčnými účinkami, imobilita, nemožnosť zmeny polohy pri riadenej a podpornej ventilácii, obezita a malnutrícia. V prípade A nastalo hojenie po vertikalizácii, zlepšovaním sebaopatery, podávaním vitamínu C so zinkom a pridávaním nutričných prípravkov (sipping), po 8 mesiacoch bol dekubitus zahojený. V prípade B (vigílna kóma) nastalo zahojenie 80 % plochy dekubitov na viacerých miestach po zavedení perkutánnej endoskopickej gastrostómie (PEG) a podávaním nutrične definovanej enterálnej výživy. V prípade C hojenie komplikovali quadruplégia a malnutrícia, pacient bol vyživovaný tekutou stravou pomocou nazogastrickej sondy. I napriek podtlakovej terapii rany nenastalo hojenie.
Záver: Vo významnej miere sme potvrdili vplyv mobility a výživy na hojenie dekubitov. Ukázalo sa, že u sledovaných pacientov je podávanie stravy nazogastrickou sondou nepostačujúce v porovnaní s PEG (podávaním nutrične definovanej enterálnej výživy).
Kľúčové slová:
dekubitus – kriticky chorí – faktory hojenia – mobilita – výživa
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors: E. Hlinková 1; J. Němcová 1; A. Simová 2; M. Balková 2
Authors place of work: Department of Nursing, Jessenius Faculty of Medicine, Comenius University, Martin, Slovakia 1; Department of Surgery and Transplantation Center, University Hospital in Martin, Slovakia 2
Published in the journal: Cesk Slov Neurol N 2018; 81(Suplementum 1): 13-18
Category: Původní práce
doi: https://doi.org/10.14735/amcsnn2018S13Summary
Aim:
The aim of the study was to identify and analyse the factors influencing the course of healing pressure ulcers in critically ill patients, then compare the variability of the healing in patients after stabilisation.
Patients and methods:
We chose a comparative multi-case study using qualitative and quantitative research methods as the research strategy. The study sample consisted of three critically ill patients with pressure ulcers.
Results:
In our study, all patients went to a critical condition after a therapeutic intervention on the heart (cases A, C – cardiac surgery; case B – radiological intervention). The pressure ulcers occurred during controlled and supportive ventilation, continuous intravenous sedation and analgesia. Hypoxia, hemodynamic instability, vasopressors with vasoconstrictive effects, obesity, malnutrition, immobility, inability to change position in controlled and supportive ventilation are factors which do not contribute to the pressure ulcers healing. In case A, healing occurred after verticalization of the patient, by improving the self-care, vitamin C with zinc and adding protein supplements (sipping). After 8 months pressure ulcer was definitely healed. In the case B (coma vigil), 80% of the area of pressure ulcers was healed after introduction to the percutaneous endoscopic gastrostomy (PEG) feeding. In case C, healing was complicated by quadriplegia and malnutrition which did not improve even by administering vitamins orally, the patient was fed via nasogastric tube. Despite negative pressure wound therapy, healing did not occur.
Conclusion:
The impact of mobility and nutrition on pressure ulcers healing was significantly confirmed in our study. It has been shown that nasogastric feeding is inadequate compared to PEG (nutritionally defined enteral nutrition).
Key words:
pressure ulcer – critically ill – factors of healing – mobility – nutrition
Introduction
Critical illness is a life-threatening condition which, in the absence of medical intervention, can lead to death or serious harm. It arises as a consequence of one or more pathophysiological processes that lead to respiratory, cardiovascular failure [1] with possible neurological damage (polyneuropathy and myopathy of critically ill) [2]. Critically ill patients require highly specialized nursing care in a technologically sophisticated environment [3]. They represent the most medically fragile and vulnerable population in the hospital, who are at high risk for developing pressure ulcers (PUs) (on a multifactorial basis). The prevalence of PUs among patients in the Intensive Care Unit (ICU)/ Department of Anesthesiology and Intensive Medicine (DAIM) is reported from 13 to 45.5% [4 – 6]. The occurrence of PUs is often unavoidable among patients in intensive and resuscita-tion care in spite of the successful implementation of the programmes for the prevention of PUs. The risk to develop PUs begins on the 1st day following the admission to ICU [7], after 15 days of hospitalisation almost all patients are at high risk of the development of PUs, especially the elderly patients [8]. Most often PUs are located in the sacrum [9,10] and buttock [11]. They are associated with mobility limitation, forced position and other constraints (chest drains, abdominal drains) in ventilated patients, which also complicate and make the healing process harder.
Aim
The aim of the study was to identify and analyse the factors influencing the course of healing PUs in critically ill patients, then compare the variability of the healing in patients after stabilisation.
Patients and methods
We chose a comparative multi-case studyusing qualitative and quantitative research methods as the research strategy (mixed methods comparatory multiple-case study). Objectivized scales for assessmentof consciousness (Glasgow Coma Scale; GCS), evaluation of agitation and sedation(Richmond Agitation and Sedation Scale; RASS), nutrition (body mass index; BMI, mini nutrition assessment; MNA), self-care (activity of daily living; ADL) were used to verify the identification of critically ill patients. The risk of PU development was assessed by Braden Scale [12]. We excluded the presence of PUs from neglect and/ or non-delivery of nursing care, so called sororigenic wounds, based on the Root Cause Analysis (RCA) as described by Pokorná et al [11]. We assessed the factors influencing wound healing based on the best available evidence, including international recommendations and management of care and treatment of PUs (age, nutrition, pharmacotherapy, physical activity). We monitored the level of PU based on the National Pressure Ulcer Advisory Panel (NPUAP) I to IV and local wound characteristics according to the University Hospital in Martin (UHM) protocol (Record of PU treatment).
The study sample consisted of three critically ill patients with PUs and the PU was acquired and formatted during hospitalisation at DAIM. All patients, after stabilisation, were dispensarised in the Outpatient Wound Care Department (OWCD) of the UHM. Sampling was deliberate.
Results
Clinical case study A. 66-year-old patient (female) with advanced cardiac failure after a cardiac bypass surgery (coronary artery bypass graft-left internal mammary artery to ramus interventricularis anterior, vena saphena magna to ramus marginalis sinister; CABG-LIMA to RIA, VSM to RMI). Pre-surgery Braden scale 11 points (high risk of PU development), with no clinical and laboratory manifestations of malnutrition. Post-surgery complicated by PU acquired. PU was diagnosed on the 5th day after admission (NPUAP I) with a tendency to grow in size and non-healing. Significant factors slowing the healing of PU were multimorbidity (hypoxia, ischemic heart disease – New York Heart Association Functional Classification III), st. p. myocardial infarction, recurrent myocardial revascularization, WHO III arterial hypertension, diabetes mellitus type II on insulin, chronic obstructive pulmonary disease), metabolic syndrome, pharmacotherapy (vasopressors), BMI 32,9 (first degree obesity) and immobility associated with supportive ventilation, deficiency of self-care in all daily activities. After 14 days the patient was transferred to the coronary unit of UHM. A PU of dimensions 22 × 15 cm (NPUAP IV) (Fig. 1), reaching depth of 2 – 3 cm when assessed by the wound care nurse, the doctor made a necrectomy. Combination of sodium hypochlorite and chlorine flushing (NaOCl/ HOCl) were recommended, charcoal with silver and iodopolyvidone activated locally according to the needs. The state of nutrition was assessed in relation to the PU treatment (MNA 12.5 points – poor nutritional state), mobility and self-care (ADL 30 points – high dependence). Recommendation: anti-decubitus mattress, repositioning, timely mobilisation and verticalization, protein supplements, sip-ping of enteral nutrition and vitamin C with zinc. During the month of treatment in UHM, the PU was reduced to 12 × 10 × 1 – 2 cm, the wound bed was only slightly coated, and gel containing NaOCl/ HOCl was locally applied in combination with 10% povidone and a sufficient absorbent layer. Daily redressing by a home care nurse, visits to the OWCD – once a month. Local treatment according to the wound healing – hydrogel, calcium alginate dressing with silver. After 10 days of treatment in home care, there was a reduction to NPUAP III, after a month reduction to 8 × 7 × 1 – 2 cm. Further treatment with alginate cover, self-adhesive foam dressing with soft silicone adhesive layer shaped for sacrum. After 5 months, PU 0.5 × 1 cm in size, only minimal secretion (Fig. 2) and after 8 months it is definitely healed.
Fig. 1. Case study A – 14th postoperative day, pressure ulcers – National Pressure Ulcer Advisory Panel (NPUAP) IV. category.
Obr. 1. Klinický prípad A – 14. pooperačný deň, dekubitus IV. stupeň podľa National Pressure Ulcer Advisory Panel (NPUAP).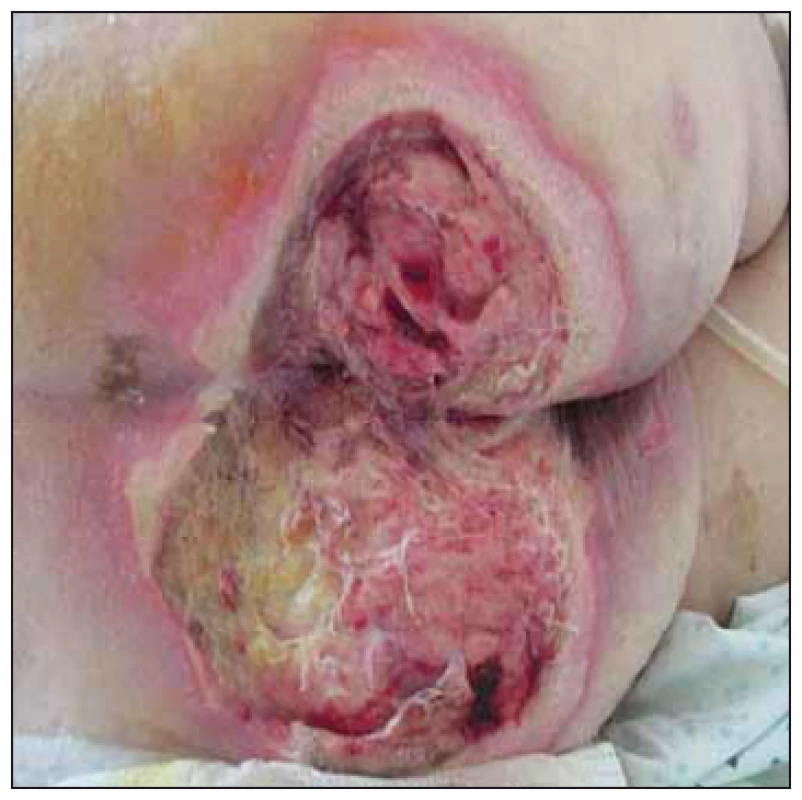
Fig. 2. Case study A – after 5 months of treatment – National Pressure Ulcer Advisory Panel (NPUAP) I. category.
Obr. 2. Klinický prípad A – po 5 mesiacoch liečby, dekubitus I. stupeň podľa National Pressure Ulcer Advisory Panel (NPUAP).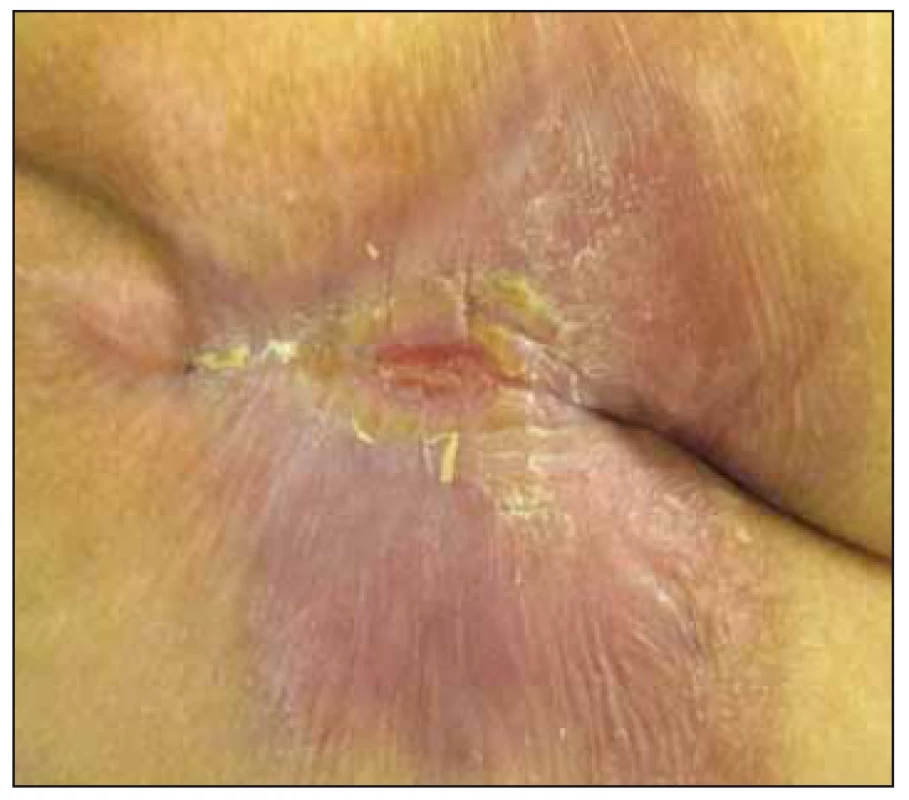
Clinical case study B. 64-year-old patient (male) with severe anoxic brain injury after C reactive protein (for malignant rhythm disorder in acute ST-Elevation Myocardial Infarction, after percutaneous coronary intervention on RIA with stent implantation, repeatedly defibrillated, intubated, admitted to DAIM for controlled ventilation. Braden scale 4 points (very high risk of PU). Persisting unconsciousness with decerebrate rigidity, quadriplegic, in coma vigil. Due to the inability to maintain airway passage, tracheostomy cannula inserted, transferred to the ICU of the Pulmonary Clinic. Fed via nasogastric tube (NGT). Wound care nurse contacted due to the occurrence of numerous PUs, mostly with necrosis: the sacral area for extensive necrosis 12 × 15 cm NPUAP non-quantifiable stage (Fig. 3), in the left trochanter 7 × 5 cm (NPUAP II), right heel necrosis 5 × 3.5 cm, outer ankle 1 × 2 cm (NPUAP II), left tibia necrosis 2 × 5 cm and 1 × 3 cm (NPUAP II), on the left hell dry furrowed skin at risk for PU, outer ankle necrosis 1.5 × 1.5 cm and the outside of shin necrosis 1 × 2 cm and 1 × 3 cm.
Fig. 3. Case study B – when patient was transferred to the Intensive Care Unit of the Pulmonary clinic, necrosis in the sacral area.
Obr. 3. Klinický prípad B – po preklade pacienta na jednotke intenzívnej starostlivosti Kliniky pneumológie, nekróza v sakrálnej oblasti.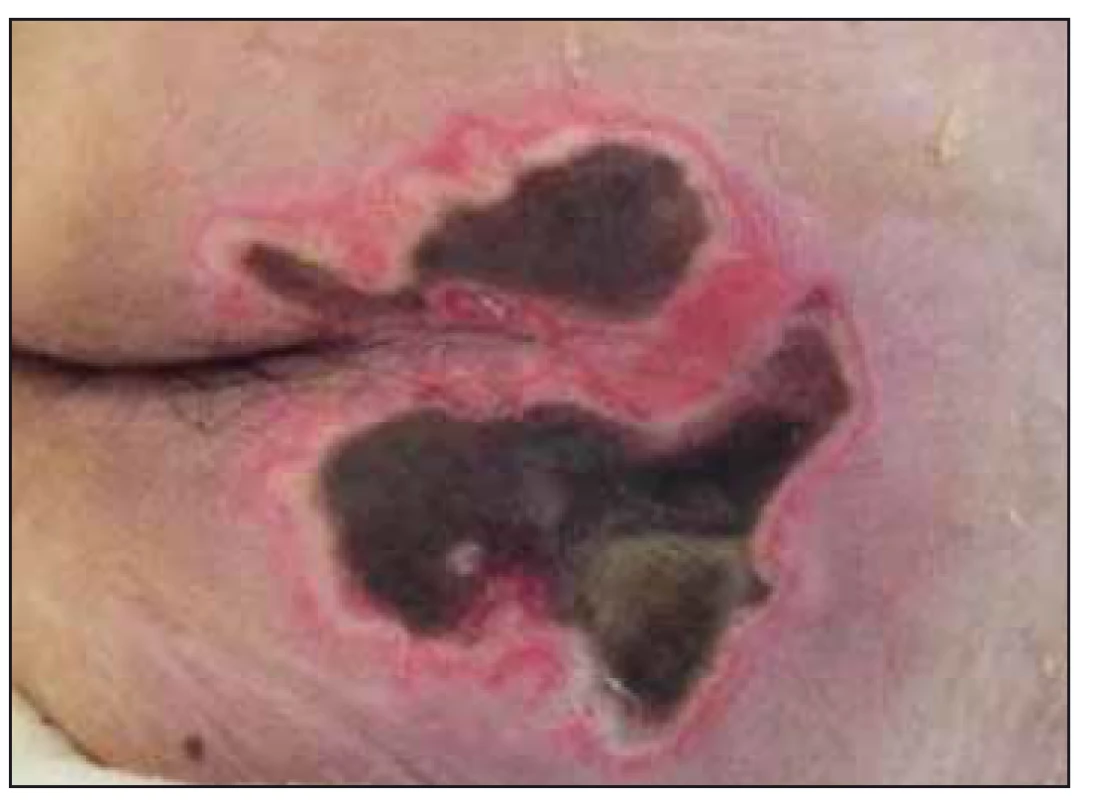
Necrectomy and debridement of all PUs performed, flushing with NaOCl/ HOCl solution, 10% povidone iodine surface treatment and deep high-absorbency fibre with reinforced fiberglass and silver dressing, sacrum and tibia with semi-permeable foaming bandage. Recommended anti-decubitus mattress, twice daily rehabilitation with a physiotherapist, enteral feeding NGT. During the 1st month of treatment healing process of PU wasinitiated, mainly in the sacral area, dimensions 10 × 10 × 1 cm (NPUAP II-III) and 9 ×7 × 1 – 2 cm (NPUAP IV) (Fig. 4) occurred in the left trochanter – significant deterioration.
Fig. 4. Case study B – the trochanteric pres sure ulcer – National Pressure Ulcer Advisory Panel (NPUAP) IV. category.
Obr. 4. Klinický prípad B – dekubitus v oblasti trochantera, IV. stupeň podľa National Pressure Ulcer Advisory Panel (NPUAP).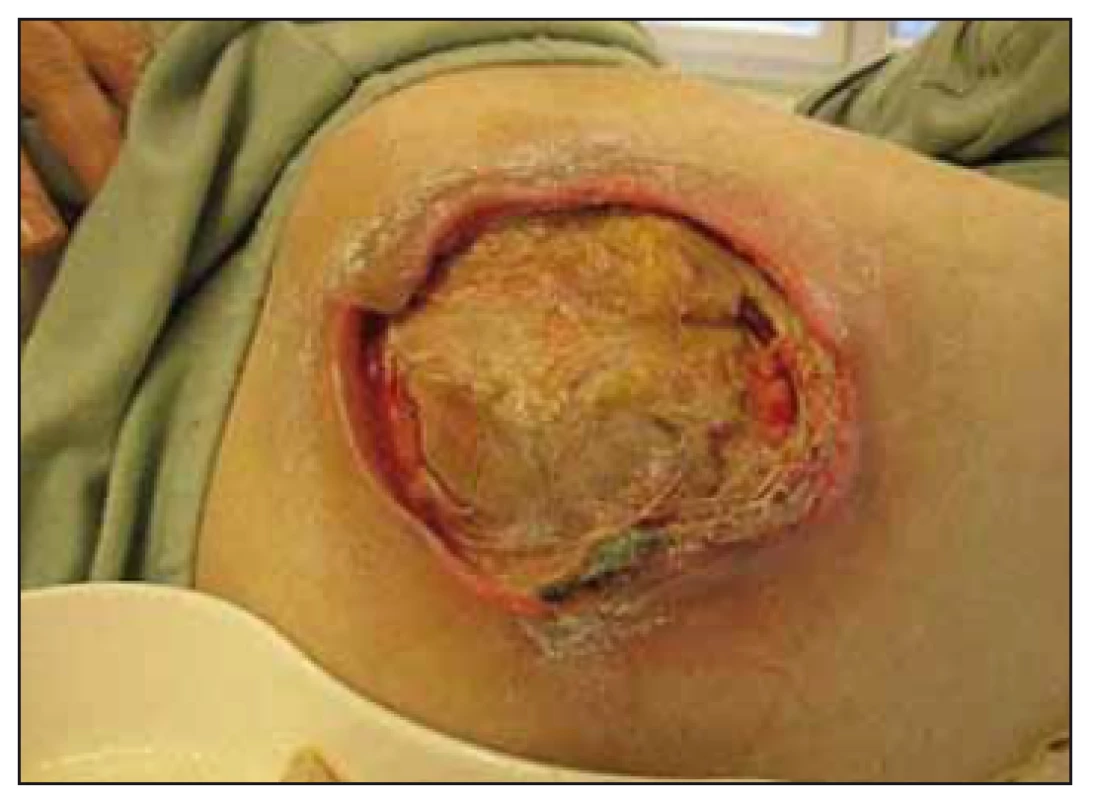
Patient released to home care, visited by a home care nurse daily. Clinical and laboratory manifestations of malnutrition (Tab. 1), thus percutaneous endoscopic gastrostomy (PEG), Flocare CH18 fy was inserted, enteral nutritional support provided (complete liquid nutrition with fibre and docosahexaenoic acid/ eicosapentaenoic acid, combined with isocaloric nutritional support for patients with diabetes or with glucose-tolerance disorders, with soluble fibre).
Tab. 1. Laboratory parameters and nutritional status in patients with pressure ulcers. 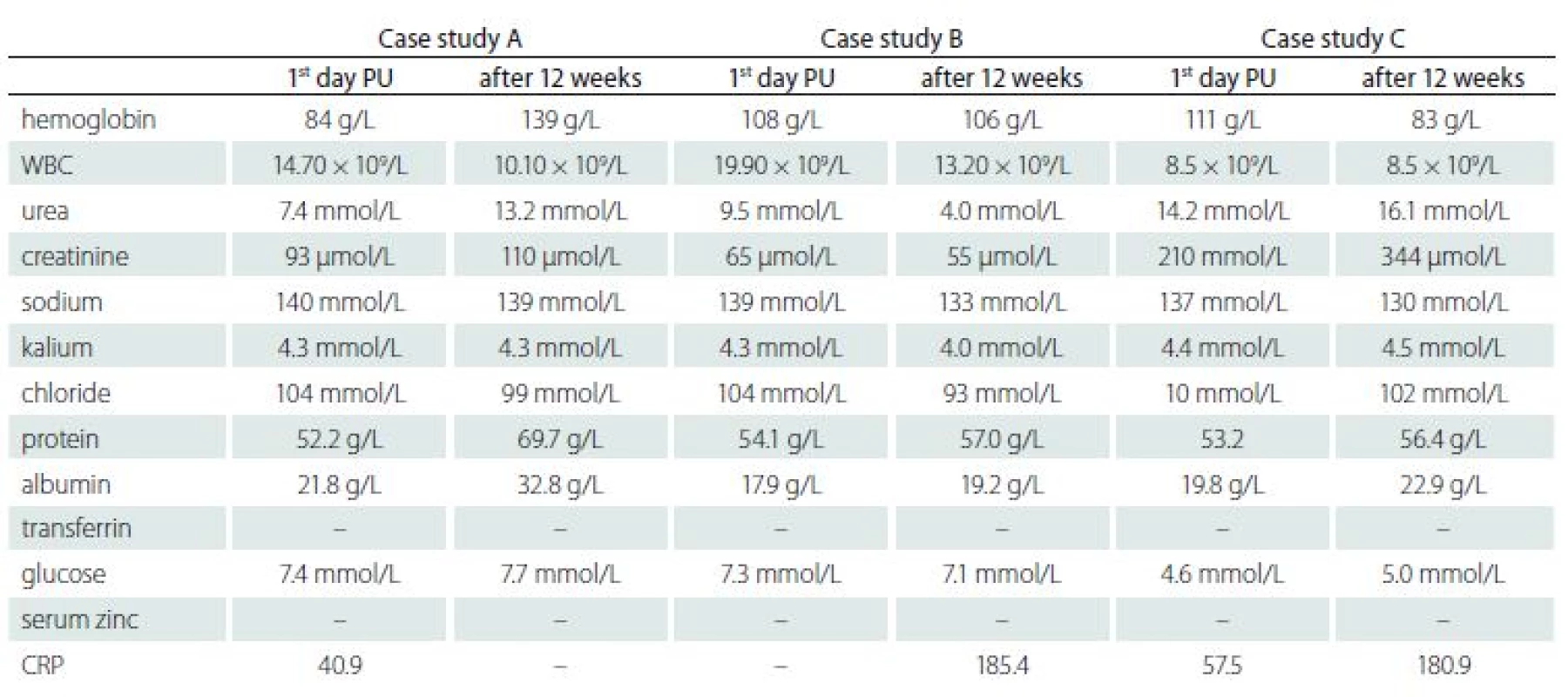
PU – pressure ulcer; WBC – white blood cell; CRP – C reactive protein – some important laboratory parameters are absent (lymphocyte, transferrin, serum zinc) Enteral nutrition administered by a continuous feeding pump for 16 h a day. Five weeks after PEG placement, a significant improvement in healing PU was identified. According to the UHM documentation, 80% of the PUs area was healed. Flushing with NaOCl/ HOCl solution recommended, surface PU to be treated with 10% povidone iodine and deep high-absorbency fibre with reinforced fiberglass and silver dressing, sacrum and tibia with semi-permeable foaming bandage. After 7 months of treatment, in the sacral area the defect of 8 × 4 cm (NPUAP II) (Fig. 5), the right trochanter healed, left 2.5 × 2.5 cm (Fig. 6), wound bed granulates. The wounds on the right heel and the right forearm healed fully. On the left tibia newly formed decubitus 2 × 2 cm and 2 × 1.5 cm, visible tendons (NPUAP IV). Flushing with polyhexanide, hydrocolloid and 10% povidone iodine locally applied. Factors decelerating the healing process – multimorbidity (arterial hypertension WHO III, ischemia, diabetes mellitus type 2 on insulin), pharmacotherapy (vasopressors), malnutrition (clinical and laboratory) and immobility in connection with controlled ventilation and subsequently in a persistent vegetative state, coma vigil.
Fig. 5. Case study B – significant healing of pressure ulcer in the sacral region after 6 months of enteral nutrition via percutaneous endoscopic gastrostomy tubes.
Obr. 5. Klinický prípad B – významné hojenie dekubitu v sakrálnej oblasti po šiestich mesiacoch podávania enterálnej výživy perkutánnou endoskopickou gastrostómiou.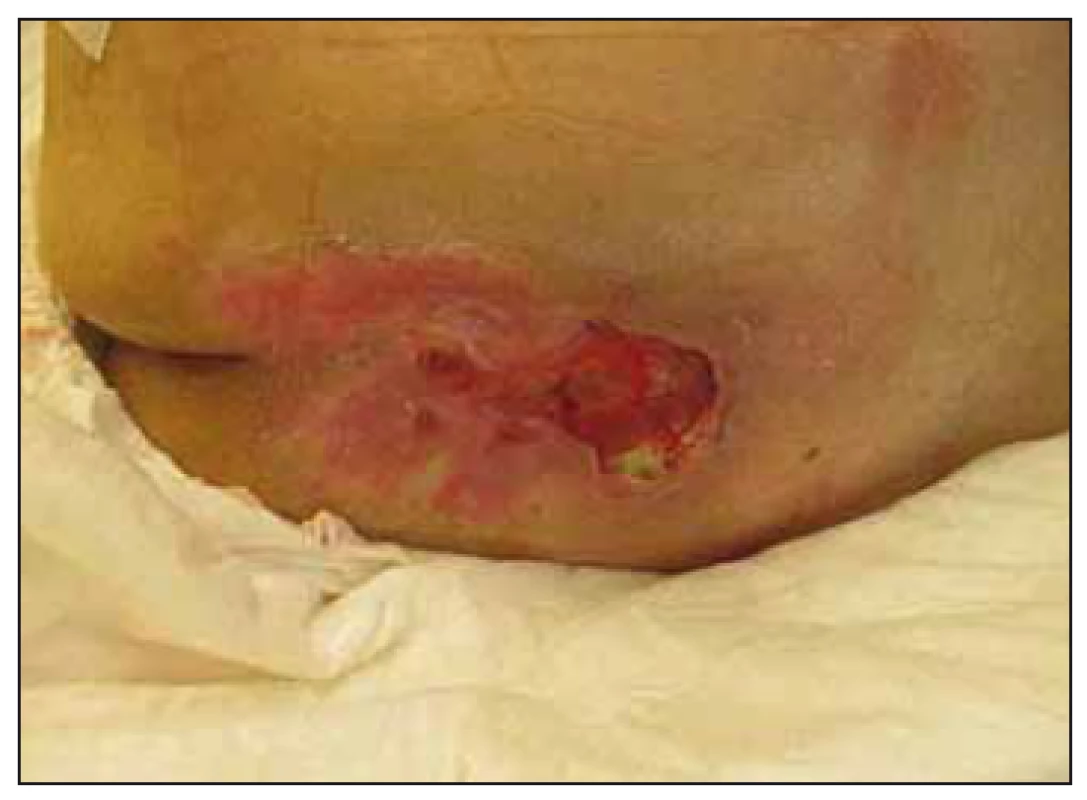
Fig. 6. Case study B – significant healing of trochanteric pressure ulcer after 6 months of enteral nutrition via percutaneous endo scopic gastrostomy tubes.
Obr. 6. Klinický prípad B – významné hojenie dekubitu v trochanterickej oblasti po šiestich mesiacoch podávania enterálnej výživy perkutánnou endoskopickou gastrostómiou.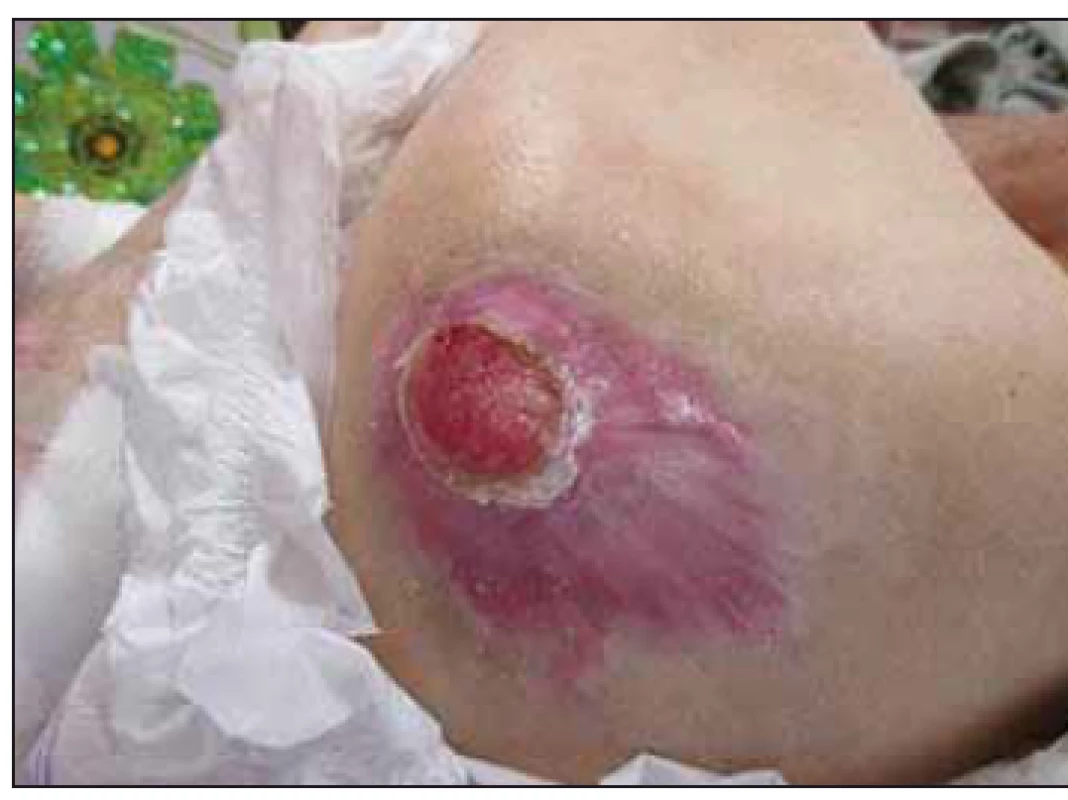
Fig. 7. Case study C – 21st postoperative day, pressure ulcer in the sacral region – National Pressure Ulcer Advisory Panel (NPUAP) IV. category.
Obr. 7. Klinický prípad C – 21. pooperačný deň, dekubitus v sakrálnej oblasti, IV. stupeň podľa National Pressure Ulcer Advisory Panel (NPUAP).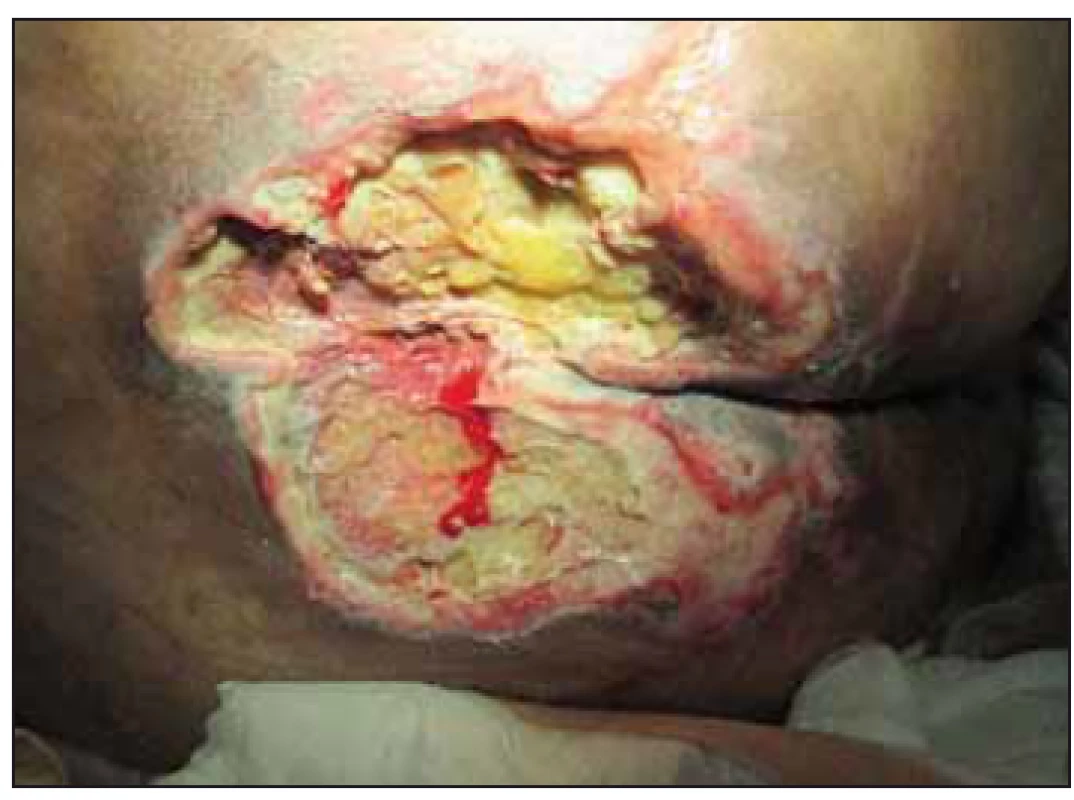
Clinical case study C. 63-year-old man hospitalized for 1 month at DAIM after car-diac surgery for severe mitral regurgita-tion (mitral valve repair). After surgerydue to respiratory failure reintubated and connected to adaptive lung ventilation (ALV). Subsequently, septic shock with renal failure, healthcare associated infection (methicillin-resistant Staphylococcus aureus), bronchopneumonia (pseudomonas aeruginosa), mediastinitis, on the 7th daysacral area PU diagnosed (NPUAP I). Afterstabilization and extubation of the patient, quadriplegia, a neurology evaluated as a brainstem lesion, polyneuropathy of critically ill patients persisted. Present additional comorbidities (dyslipoprotein-emia, heparase steatosis, chronic smoking bronchitis, benign primary arterial hypertension WHO III). A patient transferred to a local hospital with sacral PU (NPUAP IV, 15 × 15 × 2 cm, reaches the rectum, wound bed coated, min. secretion) (Fig. 7), the tracheostomy cannula inserted. Clinical and laboratory manifestations of malnutrition (Tab. 1), MNA 3.5 points (poor nutritional state), the patient is completely dependent on the help of others (ADL 0 points). OWCD performed necrectomy, enzymatic debridement, flushing with NaOCl/ HOCl solution, hydrogel sterility compression locally applied, antiseptic non-adhesive dressings from tulle fabric impregnated with 0.5% chlorhexidine acetate, non-sticky viscose dressing with honey. Repositioning, relieving, anti-decubitus mattress, vitamin C with zinc, protein supplements recommended. After 3 months, the PU was reduced (7 × 7 × 1 cm), the patient transferred to the social services house, treated outpatient for 2 months (flushing with polyhexanide, local alginate with silver), the condition did not improve, adopted on NPWT. During the stay in the social services house, new ankle bilateral ulcers (NPUAP II), treated with anatomically formed hydrocellular bandage, also appeared. A month after NPWT therapy the wound increased and deepened again (7 × 9 × 2 cm), wound bed coated, the progression of decubitus, the macerated surrounding skin. The patient was treated by nurses in the social services house and OWCD was no longer visited.
Comparison of cases
In our study, all patients went to a critical condition after a therapeutic intervention on the heart (cases A, C – cardiac surgery; case B – radiological intervention). The PUs occurred during controlled and supportive ventilation, continuous intravenous sedation and analgesia at DAIM despite the implementation of standard preventative procedures using anti-decubitus aids. NPUAP IV was diagnosed within 14 days after admission to DAIM. Hypoxia, hemodynamic instability, vasopressors withvasoconstrictive effects, immobility, inability to change position in controlled and supportive ventilation are factors which do not contribute to the PUs healing. Furthermore, in case A obesity, which complicated handling of the patient, the provision of semi-Fowler position on the bed, in cases B and C malnutrition (clinical and laboratory). The most important factors affecting PUs healing were nutrition and mobility. In case A, healing occurred after verticalization of the patient, by improving the self-care (gradually ADL 80 points – moderately self-sufficient), vitamin C with zinc and adding protein supplements to the diet (sipping nutritional supplements) until PU was healed. In case B, PEG was inserted, enteral nutrition despite the persistent vigil coma was administered. In case C, healing was complicated by quadriplegia and malnutrition which did not improve even by administering vitamins orally, the patient was fed with a slurry diet that was inadequate. Despite modern therapeutic approaches (NPWT), healing did not occur. In cases A (daughter and son) and B (wife), the patients had a great deal of support from the family, they were taken to home care. In case C, the patient was transferred to the social services home.
Discussion
Healing of a chronic wound, even in the best conditions, is a complex process that requires timely communication of cellular and extracellular components in order to restore the optimal function of the damaged tissue and also the quality of life of the individual. The quality of tissue regeneration and, in particular, the intensity of the inflammatory response may be affected by a number of factors and effects that should be analysed prior to establishing the treatment and nursing plan (continuously as well). Most authors divide these factors into system and local ones. Other divisions include internal (state of nutrition, vitamins and trace elements, tissue hypoxia, inadequate inflammatory response, immune system disorders, age of the patient, etc.) and external (infection, pharmacotherapy, devitalised tissue, physicochemical effects, etc.) Snyder et al. divided the above-mentioned factors into four categories based on the results of clinical studies: comorbidities, patient-centred factors, pharmaceuticals, micro-environment [13]. Impaired mobility to immobility exposes the individual to sustained pressure, friction [14] and so-called shear force that applies when the patient occupies Fowler‘s position when the torso „slides“ down the pad, the process of PUs healing in the sacral and sedentary areas stagnates or worsens. Insufficient food intake, poor nutrition (malnutrition) in combination with multiple co-morbidities were identified as key factors of healing PUs [15]. Randomized controlled trials have clearly highlighted the link between high-protein enteric nutrition, arginine, and vitamin C with zinc and healing of PUs [16 – 20]. The general recommendations for healing PUs include nutritional supplements ranging from 25 to 35 kcal/ kg per day [17]. All stages of healing the PUs require a sufficient intake of protein [21]. Trans Tasman Dietetic Group recommends 1.25 – 1.5 g protein/ kg body weight for patients with mild to high risk of delayed PUs healing [22]. Aging is often associated with unbalanced protein metabolism [23] and increased intake above 1.5 g/ kg per day can disrupt the nitrogen balance and cause dehydration. Therefore, it is important to monitor the hydration status and increase the intake of fluids with increased protein intake. Increased vitamin C intake with zinc is required for the wound healing process, collagen production. Zinc is a co-factor for collagen production, an antioxidant, and it is important for protein synthesis, DNA and RNA and proliferation of inflammatory cells and epithelial cells. Zinc deficiency can result in increased wound exudate. Zinc should be added, if clinical signs of zinc deficiency are present but should not exceed 40 mg per day. A high dose of zinc (> 40 mg/ day) is not recommended because it can adversely affect the copper status and may result in anaemia. If it is not possible to provide oral intake, we should provide enteral nutrition (NGT, nasojejunal tube, PEG, percutaneous endoscopic jejunostomy). According to Cochrane’s review, we should be cautious when interpreting the effects of nutrition on PUs healing. In most studies, healing of PUs was monitored by a Pressure Ulcer Scale for Healing (PUSH) score, which is not a clear objective evidence, it lacks laboratory indicators on nutrition [24]. There are other factors of healing PUs, which should be included in management of the treatment of PUs (removal of pressure, excessive moisture caused by sweat, urine and stool, treatment of infection, treatment of the primary disease, pain therapy) [25,26].
Conclusion
Critically ill patients in intensive care units are the most disadvantaged for maintaining intact skin, being at high risk, mainly due to limited mobility and physical activity. The impact of mobility and nutrition on PUs healing was significantly confirmed in our study. It has been shown that nasogastric feeding is inadequate compared to the PEG (nutritionally defined enteral nutrition). There are other factors of healing PUs, which should be included in management of the nursing care and treatment of PUs, for example tissue hypoxia, inadequate inflammatory response, immune system disorders, age of the patient, infection, pharmacotherapy, devitalised tissue, comorbidities, micro-environment etc.
Limitation of study
The study limits are sample size and only two factors influencing wound healing were root analyzed. Nutritional assessments are partial, some important laboratory parameters are absent (lymphocyte, transferrin, serum zinc). In critically ill and immobile patients, the estimation of body weight by anthropometric measures is not accurate. This supports the need for equipment to be made widely available to accurately weigh patients.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
The study was supported by the grant KEGA No. 070UK-4/2017 “The quality of nursing care for selected groups of patients”.
The study was conducted with the approval of the Ethics Commission of University Hospital in Martin. For collecting data, anonymous forms were used.
Mgr. Edita Hlinková, PhD.
Department of Nursing
Jessenius Faculty of Medicine
Malá Hora 5
036 01 Martin
Slovakia
e-mail: hlinkova@jfmed.uniba.sk
Accepted for review: 24. 6. 2018
Accepted for print: 10. 8. 2018
Zdroje
1. Robertson LC, Al-Haddad M. Recognizing the critically ill patient. Anaesth Intensive Care Med 2013; 14(1): 11 – 14. doi: 10.1016/ j.mpaic.2012.11.010.
2. Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011; 10(10): 931 – 941. doi: 10.1016/ S1474-4422(11)70178-8.
3. Cox J. Pressure injury risk factors in adult critical care patients: a review of the literature. Ostomy Wound Manage 2017; 63(11): 30 – 43.
4. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: clinical practice guideline. Haesler E (ed). 2nd ed. Perth: Cambridge Media 2014.
5. Cox J. Predictors of pressure ulcer in adult critical care patients. Am J Crit Care 2011; 20(5): 364 – 374. doi: 10.4037/ ajcc2011934.
6. Sayar S, Turgut S, Doğan H et al. Incidence of pressure ulcers in intensive care unit patients at risk according to the Waterlow scale and factors influencing the development of pressure ulcers. J Clin Nurs 2009; 18(5): 765 – 774. doi: 10.1111/ j.1365-2702.2008.02598.x.
7. Estilo ME, Angeles A, Perez T et al. Pressure ulcers in the intensive care unit: new perspectives on an old problem. Crit Care Nurse 2012; 32(3): 65 – 70. doi: 10.4037/ ccn2012637.
8. Gomes FS, Bastos MA, Matozinhos FP et al. Risk assessment for pressure ulcer in critical patients. Rev Esc Emferm USP 2011; 45(2): 313 – 318. [online]. Available from: http://www.scielo.br/ pdf/ reeusp/ v45n2/ en_v45n2a01.pdf.
9. Ahtiala MH, Soppi ET, Wiksten A et al. Occurrence of pressure ulcers and risk factors in a mixed medical-surgical ICU – a cohort study. J Intensive Care Soc 2014; 15(4): 340 – 343. doi: 10.1177/ 175114371401500415.
10. Alves PJP, Eberhardt T, Soares RSA et al. Differential diagnosis in pressure ulcers and medical devices. Cesk Slov Neurol N 2017; 80/ 113 (Suppl 1): S29 – S35. doi: 10.14735/ amcsnn2017S29.
11. Pokorná A, Saibertová S, Velichová R et al. Sorrorigenní rány, jejich identifikace a průběh péče. Cesk Slov Neurolo N 2016; 79/ 112 (Suppl 1): S31 – S36. doi: 10.14735/ amcsnn2016S31.
12. Bóriková, I. Assessment of activities of daily living. [online]. Ošetřovatelství a porodní asistence 2010; 1(1): 24 – 30. Available from URL: http:/ / periodika.osu.cz/ osetrovatelstviaporodniasistence/ dok/ 2010-01/ 4_borikova.pdf.
13. Snyder RJ, Driver V, Fife CE et al. Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: a consensus panel discussion. Ostomy Wound Manage 2011; 57(12): 36–46.
14. Vitoriano AM, Moore Z. The relationship between risk factors, risk assessment, and the pathology of pressure ulcer development. Cesk Slov Neurol N 2017; 80 (Suppl 1): S25–S28. doi: 10.14735/ amcsnn2017S25.
15. Posthauer ME, Banks M, Dorner B et al. The role of nutrition for pressure ulcer management: national pressure ulcer advisory panel, european pressure ulcer advisory panel, and pan pacific pressure injury alliance white paper. Adv Skin Wound Care 2015; 28(4): 175 – 188. doi: 10.1097/ 01.ASW.0000461911.31139.62.
16. Cereda E, Gini A, Pedrolli C et al. Disease-specific, versus standard, nutritional support for the treatment of pressure ulcers in institutionalized older adults: a randomized controlled trial. J Am Geriatr Soc 2009; 57(8): 1395 – 1402. doi: 10.1111/ j.1532-5415.2009.02351.x.
17. Dambach B, Sallé A, Marteau C et al. Energy requirements are not greater in elderly patients suffering from pressure ulcers. J Am Geriatr Soc 2005; 53(3): 478 – 482. doi: 10.1111/ j.1532-5415.2005.53168.x.
18. Ohura T, Nakajo T, Okada S et al. Evaluation of effects of nutrition intervention on healing of pressure ulcers and nutritional states (randomized controlled trial). Wound Repair Regen 2011; 19(3): 330 – 336. doi: 10.1111/ j.1524-475X.2011.00691.x.
19. Theilla M, Singer P, Cohen J et al. A diet enriched in eicosapentanoic acid, gamma-linolenic acid and antioxidants in the prevention of new pressure ulcer formation in critically ill patients with acute lung injury: a randomized, prospective, controlled trial. Clin Nutr 2007; 26(6): 752 – 767. doi: 10.1016/ j.clnu.2007.06.015.
20. van Anholt RD, Sobotka L, Meijer EP et al. Specific nutritional support accelerates pressure ulcer healing and reduces wound care intensity in non-malnourished patients. Nutrition 2010; 26(9): 867 – 872. doi: 10.1016/ j.nut.2010.05.009.
21. Lee SK, Posthauer ME, Dorner B et al. Pressure ulcer healing with a concentrated, fortified, collagen protein hydrolysate supplement: a randomized controlled trial. Adv Skin Wound Care 2006; 19(2): 92 – 96.
22. Trans Tasman Dietetic Wound Care Group. Evidence based practice guidelines for the nutritional management of adults with pressure injuries. [online]. Available from: www.guidelinecentral.com/ summaries/ evidence-based-practice-guidelines-for-the-nutritional-management-of-adults-with-pressure-injuries/ #section-date.
23. Katsanos CS, Kobayashi H, Sheffield-Moore M et al. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 2005; 82(5): 1065 – 1073. doi: 10.1093/ ajcn/ 82.5.1065.
24. Langer G, Fink A. Nutritional interventions forpreventing and treating pressure ulcers. Cochrane Database Syst Rev 2014; 12(6): CD003216. doi: 10.1002/ 1465 1858.CD003216.pub2.
25. Pokorná A, Mrázová R. Kompendium hojení ran pro sestry. Praha: Grada Publishing 2012.
26. Miertová M, Dlugošová K, Ovšonková A et al. Chosen aspects of quality of life in patients with venous leg ulcers. Cent Eur J Nurs Midw 2016; 7(4): 527 – 533. doi: 10.15452/ CEJNM.2016.07.0025.
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2018 Číslo Suplementum 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Magnosolv a jeho využití v neurologii
- Zolpidem může mít širší spektrum účinků, než jsme se doposud domnívali, a mnohdy i překvapivé
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- The factors of pressure ulcer’s healing in critically ill patients
- The pressure ulcers in patients with comorbid neurological disorders
- Factors influencing recurrence of the pressureulcers after plastic surgery – retrospective analysis
- Wound healing effects after application of polyunsaturated fatty acids in rat
- Pressure injuries prevention is better than solving of their complications
- Pressure ulcers in outpatients of the Spinal Unit of University Hospital Brno 2013– 2016
- Supraclavicular flap in reconstruction of intraoral defects
- Chondroblastic osteosarcoma of maxilla, a patient with Li- Fraumeni syndrome
- Pressure lesion monitoring – data set validation after second pilot data collection
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The pressure ulcers in patients with comorbid neurological disorders
- Chondroblastic osteosarcoma of maxilla, a patient with Li- Fraumeni syndrome
- Supraclavicular flap in reconstruction of intraoral defects
- Pressure lesion monitoring – data set validation after second pilot data collection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



