-
Medical journals
- Career
Epidemiological Study of Neurodegenerative Parkinsonism in “Hornacko”, a Specific Region of the South-eastern Moravia, Czech Republic
Authors: K. Mensikova 1; P. Kaňovský 1; P. Otruba 1; M. Kaiserová 1; M. Vastik 1; P. Hlustik 1; L. Mikulicova 1; T. Bartonikova 1; P. Dudova 1; P. Jugas 2; J. Ovecka 3; L. Sachova 4; F. Dvorský 4; J. Krsa 5; M. Godava 6; R. Vodicka 6; R. Vrtěl 6; M. Bares 7; V. Janout 8
Authors‘ workplace: Department of Neurology, Palacky University Medical School and University Hospital Olomouc 1; Neurology Outpatient Clinic, Veseli nad Moravou 2; General Practitioner, Lipov 3; General Practitioner, Velka nad Velickou 4; General Practitioner, Blatnice pod Svatym Antoninkem 5; Department of Clinical Genetics and Fetal Medicine, Palacky University Medical School, University Hospital Olomouc 6; Department of Epidemiology and Public Health, Palacky University Medical School, Olomouc 8; st Department of Neurology, Masaryk University Medical School, St. Anne University Hospital in Brno 71
Published in: Cesk Slov Neurol N 2014; 77/110(6): 714-720
Category: Original Paper
doi: https://doi.org/10.14735/amcsnn2014714Overview
Introduction:
It has been suggested that the prevalence of neurodegenerative diseases in small, isolated European communities might be higher than in the general population. We recently observed this phenomenon in a small specific region of south-eastern Moravia. Objective: To assess the prevalence of neurodegenerative parkinsonism in an isolated region with a rural population in south-eastern Moravia. Methods: A three-stage method of data collection was used. In the first phase, originally designed questionnaires were distributed to general practitioners and completed by all patients who visited them for any reason during a three-month period. In the second phase, positive responders were examined by trained primary care neurologists. Finally, the diagnosis was confirmed or excluded by a movement disorders specialist. Results: The overall prevalence in the population older than 50 years of age was 2.8% (95% CI: 2.2-3.4); the prevalence in the population from 50 to 64 years was 1.9% (95% CI: 1.2-2.5), and it was 4.06% (95% CI: 2.9-5.1) in the population over 65 years of age. Three large families with an autosomal-dominant inheritance patterns of parkinsonism were identified. Conclusions: The prevalence rates were surprisingly high; they substantially differed from the published prevalence rates in other European countries. Due to the characteristics of the region, we expected a particular impact of genetic factors, most probably the autosomal-dominant inheritance of parkinsonism. Our current research focusses on the genetic background and DNA analysis of probands from the families in which autosomal-dominant parkinsonism was identified.Key words:
parkinsonizmus – neuroepidemiologie – prevalenční studie – třístupňová vyšetřovací metoda – dědičnostIntroduction
A prevalence of the Parkinson’s disease (PD) and neurodegenerative parkinsonism is one of the highest among chronic neurological disorders. In the reports of epidemiological studies from the last two decades, the reported prevalence in Europe was approximately 1.6%, and around 1.5% in Asian and American studies [1-8]. The prevalence in the population over 65 years ranged from 1.1% to 2.2% [1-11]. Some authors also suggested that in small, isolated communities, the prevalence might be higher than in the general population [3,4,6,9].
We recently observed such a phenomenon in a remote, small, rural region (10 villages, population of 8,664, with 2,927 older than 50 years) of south-eastern Moravia, Czech Republic. We designed a pilot study during which we investigated a sample of this population from one village (population 1,524, with 230 older than 65 years) using a three-stage method of case assessment that had been successfully introduced in a recent neuroepidemiological study [3]. We found clearly increased PD prevalence with values of 2.4% (95% CI: 1.2-3.6) in the overall population, 1.7% (95% CI: 0.4-3.0) in the population between 50 and 65 years of age, and 3.5% (95% CI: 1.1-5.9) in the population over 65 years [12]. We designed and initiated an epidemiological study in the entire region, i.e., in all 10 villages, using a similar method of assessment.
Methods
Study population
The study was conducted in a small, rural, isolated region in south-eastern Moravia (Hornacko, or “Upper Lands”), a part of the Czech Republic. According to the 2011 census, 8,664 people live in this region. Low regional mobility and a traditional lifestyle are preserved in this population due to its geographic remoteness and cultural difference (Fig. 1, 2). Moravian Czech is the primary language, but the local dialect is very different from that of the surrounding villages. Hornacko has its own specific customs and traditions of folk arts and crafts that are reflected in its architecture, costumes, songs, and dances. In the past, the main working activity of this population was agriculture, cultivation and livestock; currently, the ratio of employment in agriculture, industry, and other areas do not differ from the population in other regions of the country, and diet does not differ from the rest of the Moravian region.
1. Map of Czech Republic with highlighted district borders. The dark gray area in the lower right is the Hornacko region. 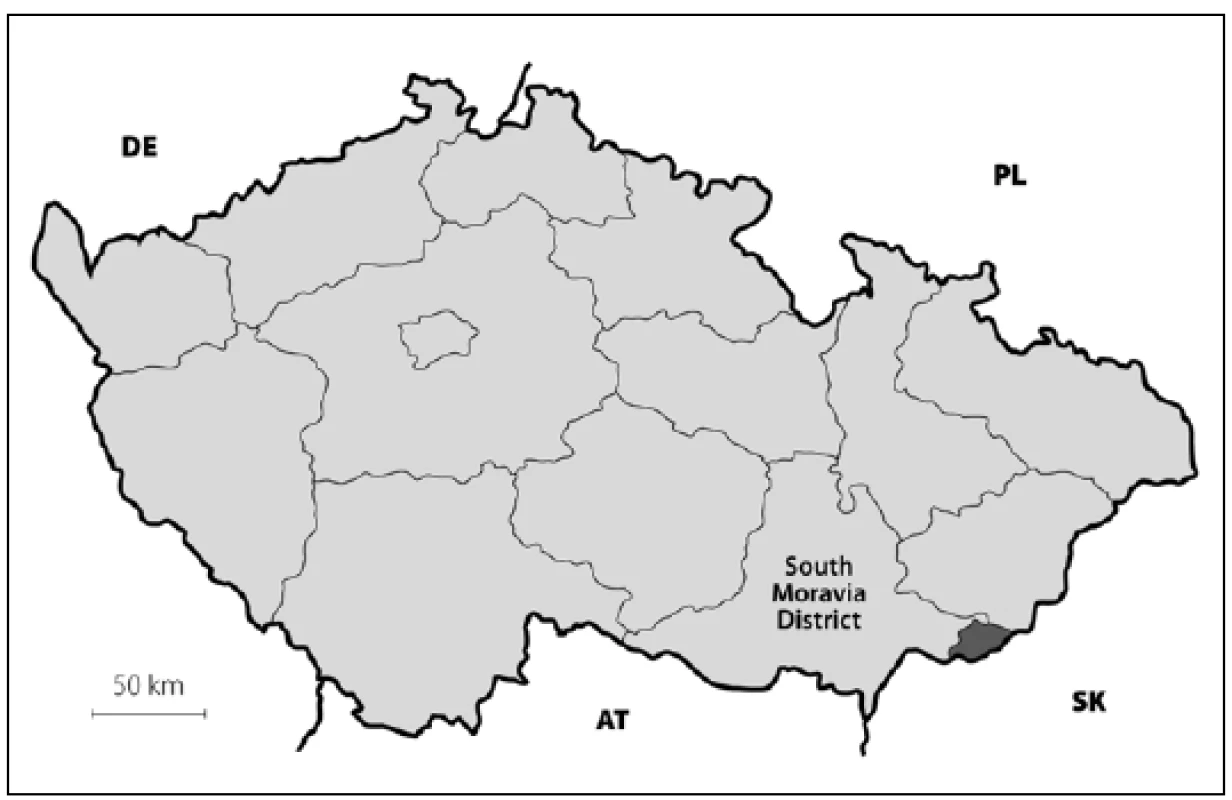
DE – Germany, PL – Poland, AT – Austria, SK – Slovakia. 2. Map of south-eastern Moravia with county border. The dark gray area is the Hornacko region, 10 villages. 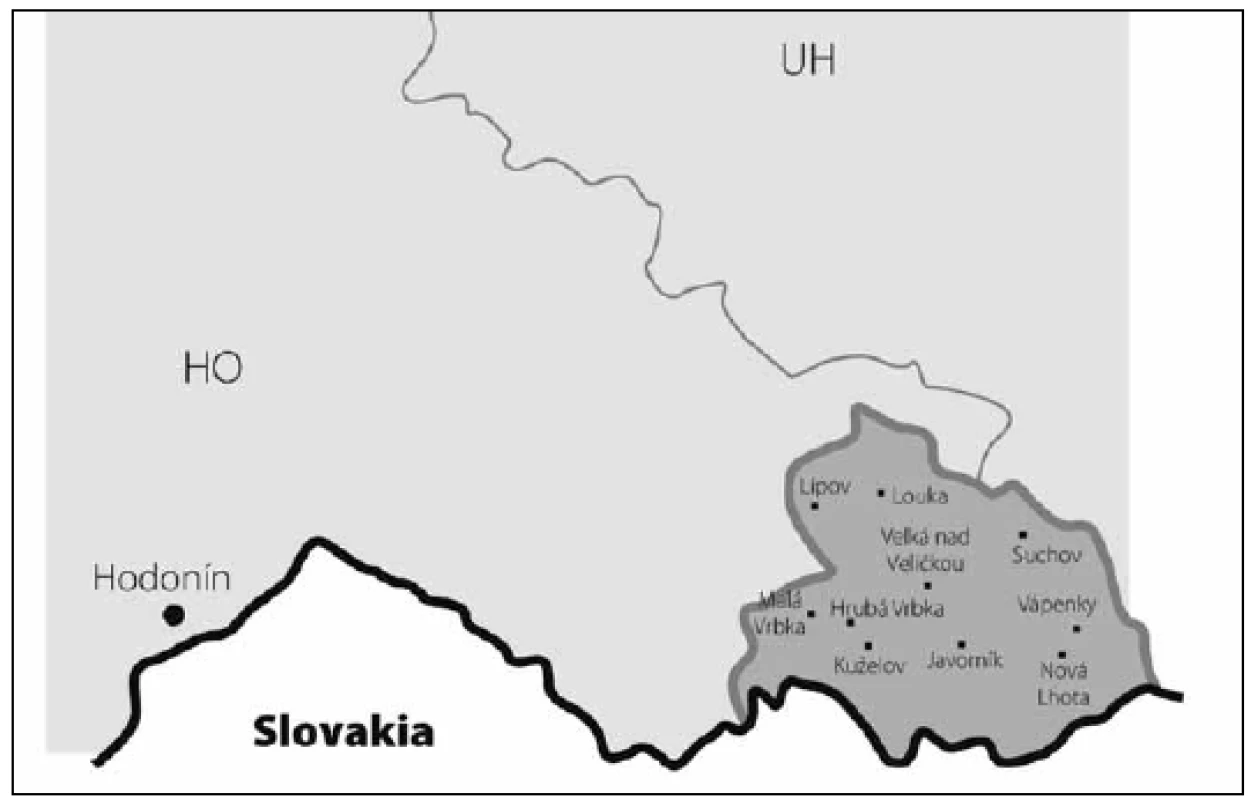
HO – Hodonin county, UH – Uherske Hradiste county, Hodonin – Hodonin county capital. Case assessment
All procedures in this study were approved by the local ethics committee of the University Hospital Olomouc. We used a three-stage case assessment method [3]. In the first stage, screening questionnaires were distributed to general practitioners; they were completed by the patients who visited the general practitioner for any reason during a three-month period. In the second stage, those who screened positively for parkinsonism according to the questionnaire and agreed to further examination (signed the informed consent) were examined by trained primary care neurologists. The motor part of the Unified Parkinson’s Disease Rating Scale (UPDRS) was completed, and a preliminary diagnosis of parkinsonism was made or rejected. In the third stage, all respondents in whom parkinsonism was suspected were admitted to the university hospital for a detailed examination in a tertiary movement disorders center, where they were examined by a neurologist with an extensive movement disorders expertise.
Appendix 1. Questionnaire (English version). 
Appendix 2. Screening log (English version). 
Screening tool
For the first phase of the study, we created a screening questionnaire that included 10 questions related to the symptoms of parkinsonism (Appendix 1). Subjects who answered positively one or more symptom questions were considered as positively screened and were invited for an examination by a trained primary care neurologist. For the second stage of the study, we developed a screening protocol consisting of 10 symptoms that may indicate the parkinsonian syndrome (Appendix 2). In this protocol, a trained primary care neurologist recorded the presence of individual symptoms of parkinsonism. Patients with two or more symptoms were invited to a specialized ward in a tertiary movement disorders center for a more detailed examination. All patients who were evaluated as positive for parkinsonian symptoms by the trained primary care neurologist in the second stage of study were re-examined by a movement disorders specialist who did not participate directly in the study.
Diagnostic criteria
In the third stage of the study, parkinsonism was diagnosed when at least two of four symptoms (resting tremor, rigidity, bradykinesia, and impaired postural reflexes) were present in a subject not receiving antiparkinsonian medication. During hospitalization, all patients were examined in detail to exclude secondary forms of parkinsonism. The examination protocol included neurological, psychological, and psychiatric examinations, examination of cerebrospinal fluid and neuroimaging (magnetic resonance imaging of the brain, dopamine transporter imaging - DATscan, perfusion SPECT); the neurophysiological examinations (electroencephalographic recordings, electromyography, and assessments of visual, auditory, somatosensory, and motor evoked potentials) were also done.
Primary care neurologist training
Training of primary care neurologists was conducted by movement disorders specialists and was focused on phenomenology of parkinsonism. Emphasis was on a patient history and neurological examination, including the motor part of the UPDRS. Training was conducted prior to the study, also using case studies and video presentations; several cases were discussed. At the end of the training, the primary care neurologists were required to correctly assess the motor parts of the UPDRS scales in two parkinsonian patients.
Data analysis
Prevalence was calculated by age group as the number of cases relative to the number of region inhabitants.
Epidemiological analysis
All patients admitted for the confirmation of diagnosis to the tertiary movement disorders center were examined by a clinical geneticist. They were asked in detail about the family history of neurological disease, particularly parkinsonism, and they were encouraged to build their family tree. They were assisted by trained members of movement disorders center staff. In the next step, these trees were completed; for this part of work, parish registers in all villages (and older ones stored in the Moravian and Lower Austrian Land Archives) were used for extraction of births, marriages and deaths files. Completed family trees were then analyzed by a blinded clinical geneticist.
Results
The study population consisted of 8,664 inhabitants of the region; of these, 2,927 subjects were 50 years of age and older: 1,672 inhabitants aged 50-64 years, and 1,255 inhabitants aged 65 years and older [13].
A total of 2,200 screening questionnaires were delivered to general practitioners. Of these, 1,167 completed questionnaires were returned to the general practitioners; response rate was 53.04%.
In the first stage, 247 (21.17%) respondents screened positive for parkinsonism on the questionnaire, experiencing one or more symptoms. In the second stage, positively screened respondents were invited for an examination at the general practitioner’s office. A trained primary care neurologist made a diagnosis of possible parkinsonism in 88 subjects (35.62% of the positive respondents).
In the third stage, final neurological examination by a movement disorders specialist confirmed the preliminary diagnosis of parkinsonism in 83 patients (33.6% of the initially positive respondents). Of the 88 patients who had screened positive for signs of parkinsonism in the second stage, two patients (2.2%) were diagnosed with essential tremor and three patients (3.5%) had no parkinsonian signs. The group of patients with confirmed parkinsonism and older than 50 years consisted of 35 males, whose mean age was 68.9 years and 48 females with a mean age of 65.6 years. Of these, 13 males and 19 females aged 50-64 years and 22 males and 29 females were aged 65 years or more. The presence and distribution of initial parkinsonian signs in different age groups are shown in Tab. 1.
1. Number of patients in different age groups and presence of clinical signs of parkinsonism. 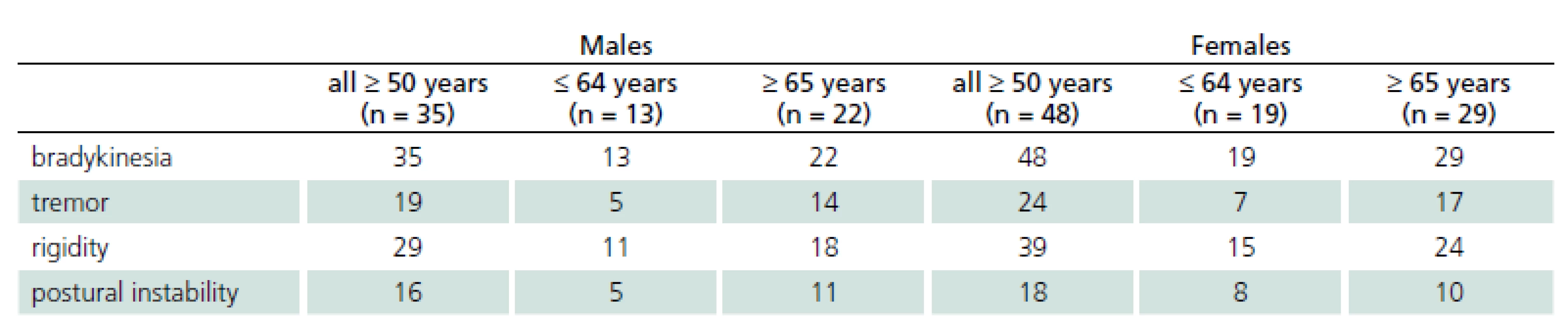
The prevalence rates were calculated by an epidemiological statistician using data from the Czech Statistical Office [13]. Overall prevalence in the population older than 50 years was 2.8% (95% CI: 2.2-3.4). The prevalence in the population from 50 to 64 years of age was 1.9% (95% CI: 1.2-2.5) and it was 4.06% (95% CI: 2.9-5.1) in the population over 65 years of age.
Three large families with familial occurrence of parkinsonism were found that encompassed inhabitants of all researched villages. Upon the final analysis of completed family trees, the blinded clinical geneticist identified clear autosomal-dominant inheritance pattern in all of them. These family trees are shown in the Fig. 3-5.
3. Pedigree Nr. 1 – HorPark 1. 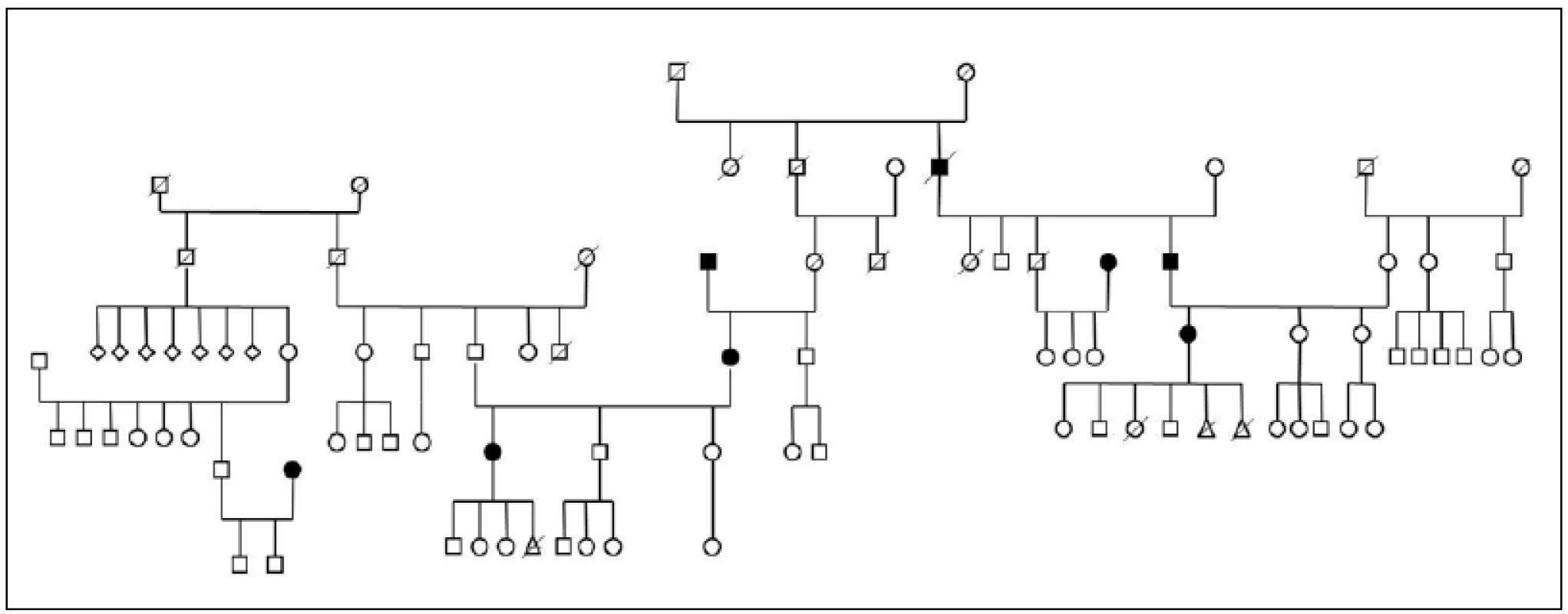
Full circles and squares: probands with diagnosed parkinsonism. 4. Pedigree Nr. 2 – HorPark 2. 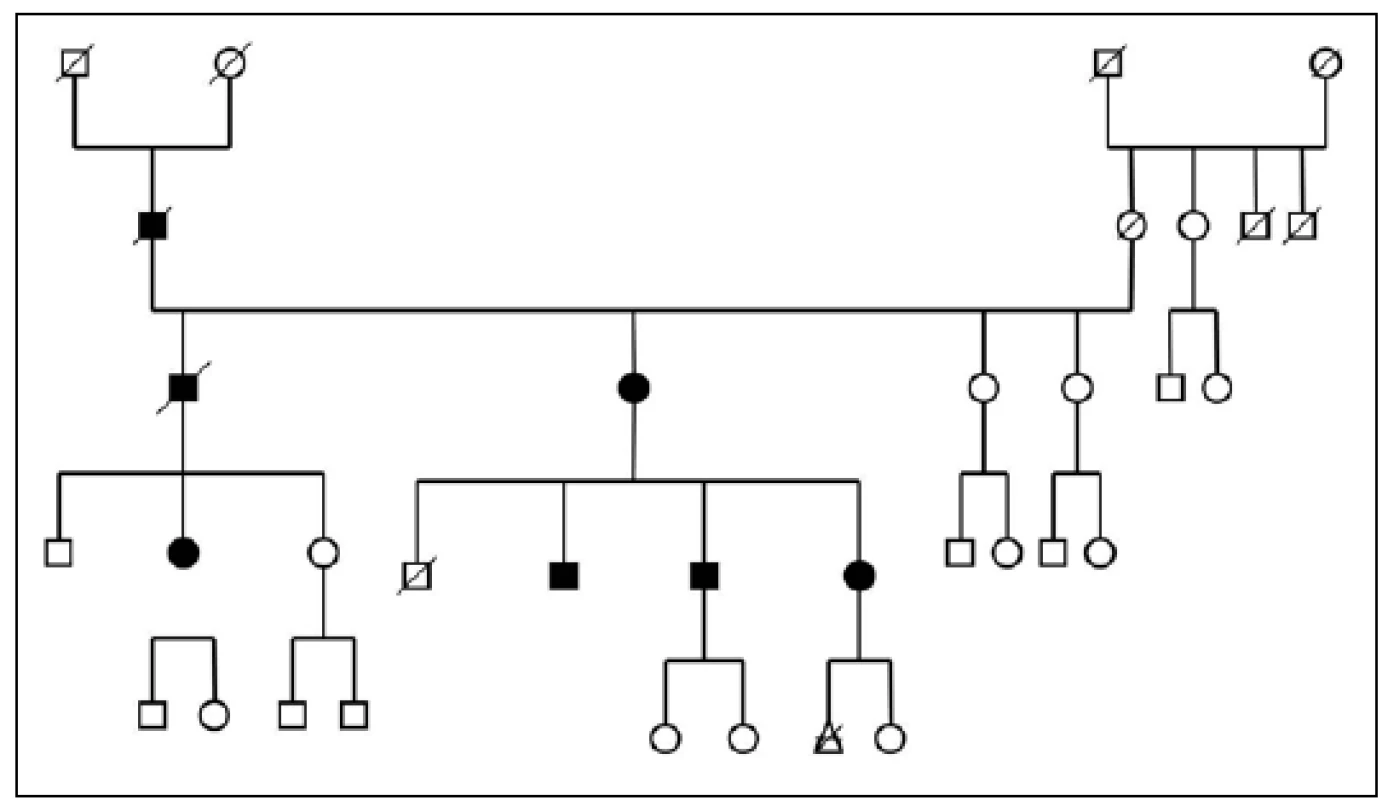
Full circles and squares: probands with diagnosed parkinsonism. 5. Pedigree Nr. 3 – HorPark 3. 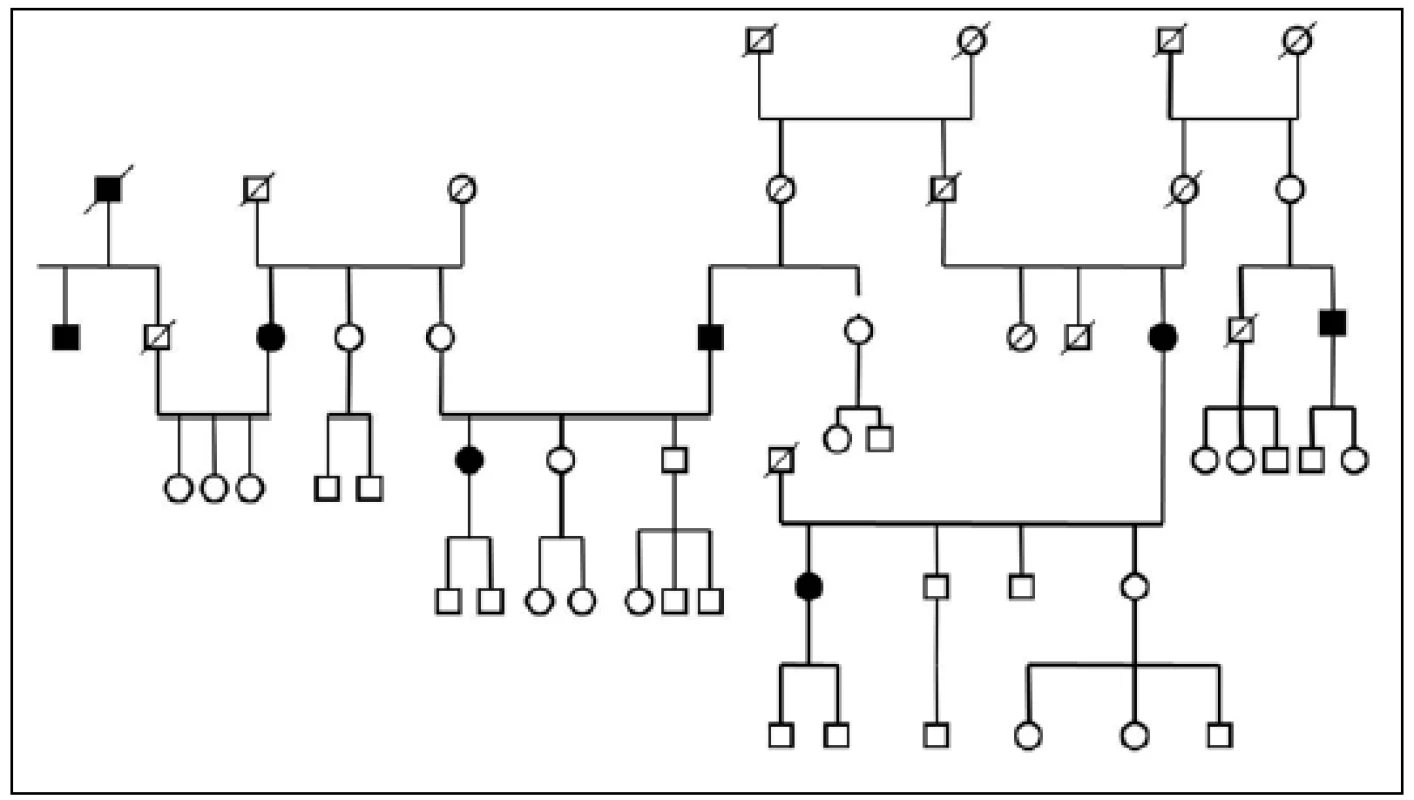
Full circles and squares: probands with diagnosed parkinsonism. Discussion
Various investigations of the prevalence of PD and parkinsonism among different populations have been conducted. The wide range of prevalence estimates that have been reported in various populations (from 10 to 405 cases per 100,000) may result from different genetic factors or different environmental factors, or both [5]. The range may also be the result of different assessment methods or different diagnostic criteria. For epidemiological surveys, the three most important methodological elements are the case-finding strategy, diagnostic criteria, and degree of coverage of the target population [1]. Several door-to-door population surveys were recently completed and published in order to establish the prevalence of parkinsonism or Parkinson’s disease worldwide [8,10,11]. The door-to-door survey is probably the best way to estimate the prevalence of a disease in a population [5]. However, it is a very costly and labor-intensive method [3]. On the other hand, community studies that are based on case detection through physician or hospital visits yield false lower prevalence rates, despite the use of homogeneous diagnostic criteria [14]. The results may also be jeopardized by the diagnostic criteria used for the research. The PD prevalence rate in our survey may be affected by the criteria used for clinical diagnosis. Using the Gelb, Oliver and Gilman criteria, in which bradykinesia is not a required feature of PD, we could get a different prevalence rate than with the more commonly used UK-PDBB criteria [15-17]. For these reasons, we adopted a three-stage method of case assessment for our pilot and the present study; a methodology similar to one that was successfully used to investigate the prevalence of parkinsonism and Parkinson’s disease in South Tyrol [3].
The prevalence of Parkinson’s disease or neurodegenerative parkinsonism has not yet been investigated in any of the Central European countries. Both our surveys (pilot and presented) have been completed in one of the most specific areas of the Czech Republic and the entire Central Europe as emphasized in our preliminary reports [12]. Hornacko (“Upper Lands”, Fig. 1, 2) is formed by 10 villages. According to the Czech Statistical Office, its total population in 2012 was 8,664 people [13]. Hornacko is a small autonomous geographical entity in the White Carpathian Mountains (villages are at up to 500 m above the sea level, the mountains are up to 950 m in height); its geographical borders are formed by two valleys that create the catchment area for two small rivers, the Velicka and Kuzelov stream. Geographical borders have not changed for the last 200 years. Nevertheless, the area has historically been primarily defined ethnographically. The original Slavic population that lived here from the early 9th century was a frequent target of raids during the Hungarian and Turkish invasions (the villages’ districts formed in fact the border with the Hungarian kingdom); the last Turkish occupation dates to the early 18th century. As a result, this area remained practically uninhabited, and was newly colonized in the mid-18th century, mostly by Lutherans originating mainly in Silesia, western Slovakia, Burgenland and Styria. These people settled in the remnants of the original Slavic villages and eventually merged to create a homogeneous population, specific and distinct from the surrounding ancient Moravian population in terms of language dialect, daily life customs, costumes, folk music, and other traditions, including dating and marriage. One important fact is that marriages outside the area of 10 villages were (and they still are) rare; this situation probably caused the relative genetic homogeneity that has been only fractionally altered over the course of the last two centuries [18-21]. The question is whether the apparently higher prevalence of neurodegenerative parkinsonism in this population reflects its original genetic background, or whether it is a result of relative social (and therefore also genetic) isolation and subsequent high level of in-breeding. Three family trees (Fig. 3-5) in which the autosomal-dominant inheritance pattern of parkinsonism was revealed would rather support the latter hypothesis; nevertheless, the prevailing Lutheran faith in all three families would speak in favor of the former. Our detailed genealogical analysis also revealed the origin of all three families in one of the villages (Javornik, PCN 69674, 350 m above the sea level, 720 inhabitants). This village is almost entirely inhabited by people of Lutheran faith and, according to the historical records, this faith prevailed in the village for the last 200 years. The weak point of this hypothesis is the lack of any valid documentation of the origins of the population of this village; according to the historical tradition, the majority of people came in the mid 18th century from central and western Slovakia (then a part of Hungarian kingdom).
In the past, some studies have also suggested that people living in rural areas could be more exposed to putative environmental - mainly agricultural - influences and would be more likely to develop parkinsonism than those living in towns [22]. However, from our point of view, this hypothesis seems to be a pure speculation. The surveyed area is a part of a much larger rural area where agricultural and environmental conditions are quite similar; up to now, no signs of higher incidence of parkinsonism in this (or any other) part of the Czech Republic have ever been observed or reported. Therefore, the question of the origins of such a high parkinsonism and PD prevalence remains unanswered.
Undoubtedly, a molecular-genetic analysis will be done in all probands in whom the parkinsonian symptoms were confirmed, followed by analyses in their co-sanguine relatives. Nevertheless, we also plan to continue with environmental investigations, as this hypothesis must also be confirmed or rejected.
Acknowledgment
The authors are grateful to Jan Pavlik, MD, native of Kuzelov, Hornacko researcher, writer, singer and dancer, for his substantial contribution to the manuscript by providing ethnographic data and references.
Funding support: The study was supported by the grants IGA MZ CR NT – 14407-3/2013 and IGA LF UP – 2012–005 and 2013–24, and by the Ministry of Health, Czech Republic, conceptual development of research organization – Nr.1 RVO-FNOL2013.
This paper is dedicated to PhC. Jana Pavlíková-Fialová (1961–1985).
Katerina Mensikova, MD, PhD
Department of Neurology
Palacky University Medical School and University Hospital
I. P. Pavlova 6
775 20 Olomouc
e-mail: katmen@centrum.cz
Accepted for review: 9. 1. 2014
Accepted for print: 20. 1. 2014
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Sources
1. de Rijk MC, Tzourio C, Breteler MM, Dartigues JF, Amaducci L, Lopez-Pousa S et al. Prevalence of parkinsonism and Parkinson’s disease in Europe: the EUROPARKINSON Collaborative Study. J Neurol Neurosurg Psychiatry 1997; 62(1): 10-15.
2. de Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, van der Meche FG et al. Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology 1995; 45(12): 2143-2146.
3. Kis B, Schrag A, Ben-Shlomo Y, Klein C, Gasperi A,Spoegler F et al. Novel three-stage ascertainment method: prevalence of PD and parkinsonism in South Tyrol, Italy. Neurology 2002; 58(12): 1820-1825.
4. Benito-León J, Bermejo-Pareja F, Rodríguez J, Molina JA,Gabriel R, Morales JM. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov Disord 2003; 18(3): 267-274.
5. Zhang ZX, Román GC. Worldwide occurrence of Parkinson’s disease: an updated review. Neuroepidemiology 1993; 12(4): 195-208.
6. Barbosa MT, Caramelli P, Maima DP, Cunningham MC, Guerra HL, Lima-Costa MF et al. Parkinsonism and Parkinson’s disease in the elderly: a community-based survey in Brazil (the Bambuí study). Mov Disord 2006; 21(6): 800-808.
7. Das SK, Misra AK, Ray BK, Hazra A, Ghosal MK, Chaudhuri A et al. Epidemiology of Parkinson disease in the city of Kolkata, India: a community-based study. Neurology 2010; 75(15): 1362-1369. doi: 10.1212/ WNL.0b013e3181f735a7.
8. Winkler AS, Tutuncu E, Trendafilova A, Meindl M, Kaaya J, Schmutzhard E et al. Parkinsonism in a population of northern Tanzania: a community-based door-to-door study in combination with a prospective hospital-based evaluation. J Neurol 2010; 257(5): 799-805. doi: 10.1007/ s00415-009-5420-z.
9. Wermuth L, Joensen P, Bunger N, Jeune B. High prevalence of Parkinson’s disease in the Faroe Islands. Neurology 1997; 49(2): 426-432.
10. Bergareche A, De La Puenta E, López de Munain A,Sarasqueta C, de Arce A, Poza JJ et al. Prevalence of Parkinson’s disease and other types of Parkinsonism. A door-to-door survey in Bidasoa, Spain. J Neurol 2004; 251(3): 340-345.
11. Clavería LE, Duarte J, Sevillano MD, Pérez-Sem-pere A,Cabezas C, Rodriguez F et al. Prevalence of Parkinson’s disease in Cantalejo, Spain: a door-to-door survey. Mov Disord 2002; 17(2): 242-249.
12. Mensikova K, Kanovsky P, Kaiserova M, Mikulicova L, Vastik M, Jugas P et al. Prevalence of neurodegenerative parkinsonism in an isolated population in south-eastern Moravia, Czech Republic. Eur J Epidemiol 2013; 28(10): 833-836. doi: 10.1007/ s10654-013-9823-x.
13. Český statistický úřad. Statistická ročenka České republiky 2011. Dostupné z URL: http:/ / czso.cz/ csu/ 2011edicniplan.nsf/ publ/ 0001-11-2010.
14. Anderson DW, Rocca WA, de Rijk MC, Grigoletto F,Melcon MO, Breteler MM et al. Case ascertainment uncertainties in prevalence surveys of Parkinson’s disease. Mov Disord 1998; 13(4): 626-632.
15. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999; 56(1): 33-39.
16. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55(3): 181-184.
17. de Rijk MC, Rocca WA, Anderson DW, Melcon MO,Breteler MM, Maraganore DM. A population perspective on diagnostic criteria for Parkinson’s disease. Neurology 1997; 48(5): 1277-1281.
18. Frolec V, Holý D, Jeřábek R. Hornacko. The life and culture of the people on the Moravia-Slovakia border in the White Carpathian area. Brno: Blok 1966.
19. Pavlicová M. Folk culture and its historic and social reflexes. Brno: Masaryk University Press 2007.
20. Válka M. Social and cultural changes in the village: moravian countryside at the turn of third millennium. Brno: Masaryk University Press 2011.
21. Pavlík J. Kuzelov - Hornacko village under the sails of a windmill. Kuzelov: Obec Kuzelov 2005.
22. Koller W, Vetere-Overfield B, Gray C, Alexander C, Chin T, Dolezal J et al. Environmental risk factors in Parkinson’s disease. Neurology 1990; 40(8): 1218-1221.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2014 Issue 6-
All articles in this issue
- Reducing the Door-to-needle Interval, Experience from the Stroke Centre in Kladno
- WHO Grade II Ependymomas of the Fourth Ventricle in Adults – Single Institution Experience
- Diazepam i. m. – the Most Common, but Inappropriate Medication for Management of Acute Anxiety, Agitation and Aggression
- Sporadic Use of Decompressive Hemicraniectomy in a Patient with Brain Abscess – a Case Report
- Missile Head Injury with a Replica of a Historical Weapon – Pathophysiology and a Case Study
- Adult Form of Pompe Disease
- Cognitive Rehabilitation in Patients with Multiple Sclerosis
- Effectiveness of the Temporary Splinting after Carpal Tunnel Release
- Increasing the Sensitivity of Brain Death Confirmation Using the Combination of Auditory and Somatosensory Evoked Potentials
- The Five Point Test – a Test of Nonverbal Fluency: Normative Data for Adults
- Molecular Diagnostics of NF1 in Slovakia Using cDNA and MLPA Analysis
- The Effect of Suboptimal Surgical Treatment of Spinal Fractures on the Course of Spinal Cord Injury
- The Use of Electromagnetic Navigation in Surgeries for Hydrocephalus and Arachnoid Cysts in Children under One Year of Age
- Is Electrophysiology Useful in the Differential Diagnostics of Lumbar Spinal Stenosis and Diabetic Polyneuropathy?
- Epidemiological Study of Neurodegenerative Parkinsonism in “Hornacko”, a Specific Region of the South-eastern Moravia, Czech Republic
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Diazepam i. m. – the Most Common, but Inappropriate Medication for Management of Acute Anxiety, Agitation and Aggression
- Missile Head Injury with a Replica of a Historical Weapon – Pathophysiology and a Case Study
- The Five Point Test – a Test of Nonverbal Fluency: Normative Data for Adults
- Adult Form of Pompe Disease
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

